SCRI AMS GRADE Methodology for Clinical Practice Guidelines 2024
|
Programme Duration: |
2 May – 11 June 2024 |
|
Venue: |
CRIS Office and Virtual |
|
Organisers: |
Cochrane Singapore, Academy of Medicine Singapore (AMS), and Singapore Clinical Research Institute (SCRI) |
In May and June 2024, SCRI, Cochrane Singapore and
the Academy of Medicine, Singapore (AMS) jointly organised a workshop designed
to provide AMS workgroup members and clinicians with the technical
skills and knowledge to develop their own clinical practice guidelines
(CPGs). CPGs are important for patient care, because these frameworks ensure
consistent, evidence-based care to support healthcare professionals in
making informed and effective treatment decisions. The training focused
on effectively utilising the Grading of Recommendations Assessment, Development
and Evaluation (GRADE) guidelines methodology to support evidence-based
framework to support clinical decision making.
The workshop consisted of three modules over seven sessions, covering key aspects such as:
-
Introduction to the clinical practice guideline development process
-
Technical skills in collecting evidence, synthesis and reporting
-
Strategies to transition from evidence to recommendation
The workshop drew 76 AMS members from across the public healthcare institutions, who also provided positive feedback by the end of the course.

Module 1 – Introduction to the clinical practice guideline development process
2 May 2024, Virtual
Module 1 of the workshop began with a welcome address by A/Prof Danny Soon, Chief Executive Officer, Consortium for Clinical Research and Innovation Singapore and Executive Director, SCRI.
During the session, participants grasped a clear understanding of how the GRADE guidelines development methodology. will steer clinicians towardsevidence-based and actionable Clinical Practice Guidelines for improved care outcomes.
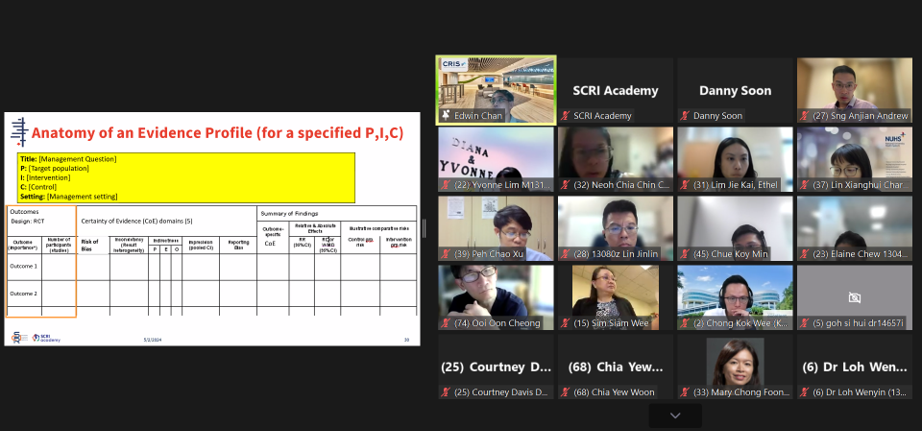
A/Prof Edwin Chan, Chief Scientific Officer, SCRI and Director, Cochrane Singapore, explaining the various components of an evidence profile used in developing GRADE guidelines.
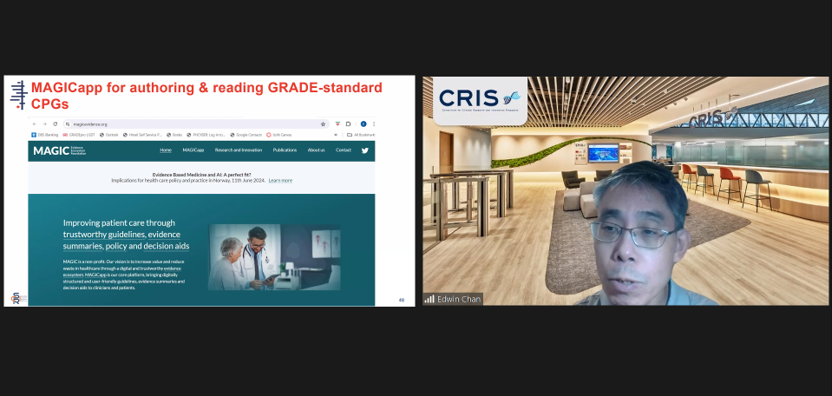
A/Prof Edwin Chan, Chief Scientific Officer, SCRI and Director, Cochrane Singapore, introducing the MAGICapp as a tool for participants to conduct evidence-based research on Clinical Practice Guidelines.
Module 2 – Technical skills in evidence collation, synthesis and reporting
Part 1 – 7 & 8 May 2024, Virtual
Part 2 – 15 & 16 May 2024, CRIS Office
In the first part of Module 2, conducted virtually, participants delved into the theoretical aspects of conducting a literature search. They also learnt the crucial skills needed for the critical appraisal of research data and evidence synthesis, essential for developing therapeutic, prognosis and diagnosis guidelines.
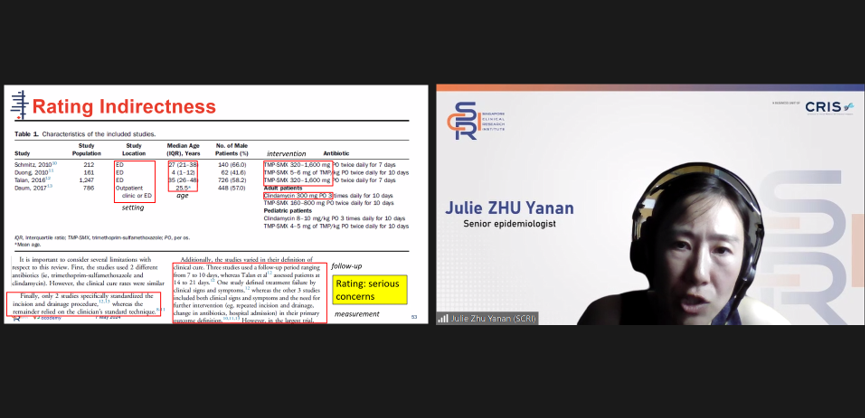
Dr Julie Zhu, Senior Epidemiologist, guided the class in how to interpret data found in clinical practice research papers.
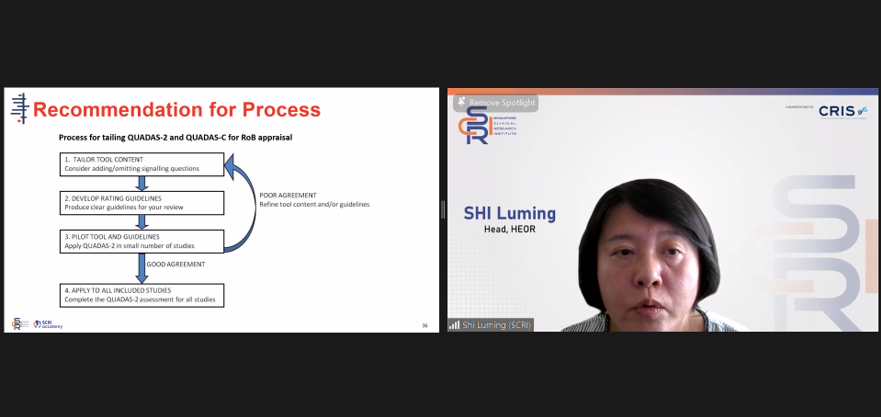
Dr Shi Luming, Head of Health Economics and Outcomes Research (HEOR), SCRI, provided clear explanation and analogies to support the current methodology for the recommendation process of clinical decision-making.
In the latter half of Module 2, participants could engage in group work and lively discussions to show how well they understood the concept and could effectively apply tools such as GRADEproGDT and MAGICapp to collate evidence from eligible studies and generate a Summary of Findings (SoF) table for further guideline development. The session also provided participants with hands-on experience to reinforce their newly acquired skills through practice scenarios.


From the top left: Dr Julie Zhu, guiding a participant during the demonstration segment. A/Prof Edwin Chan, Dr Julie & Dr Guo Liang answering questions from participants after the workshop.
Module 3 – Transforming evidence into actionable recommendations
Part 1 – 11 June 2024 (AM), Virtual & CRIS Office
Part 2 – 11 June 2024 (PM), CRIS Office
The final module focused on applying earlier collated evidence to achieve a consensus for formal recommendations, ultimately culminating in a complete clinical practice guideline.
The first segment of the module introduced participants to the theoretical aspects of the GRADE evidence-to-decision framework, illustrating how the framework could enhance consensus building among panels. Next, participants were given the opportunity to actively engage in informing recommendations within their respective workgroups.
Participants were able to translate evidence from ten pre-specified outcomes into weak recommendation in using corticosteroids in addition to standard care in patients with sore throat. The conclusion was derived from the tradeoff between the benefits and harms of the treatment, taking consideration of other key elements such as certainty of evidence, values and preference, and also resources.

We would like to thank all participants for their enthusiasm throughout this workshop!
This event is jointly presented by Cochrane Singapore, Singapore Clinical Research Institute and Academy of Medicine, Singapore.

